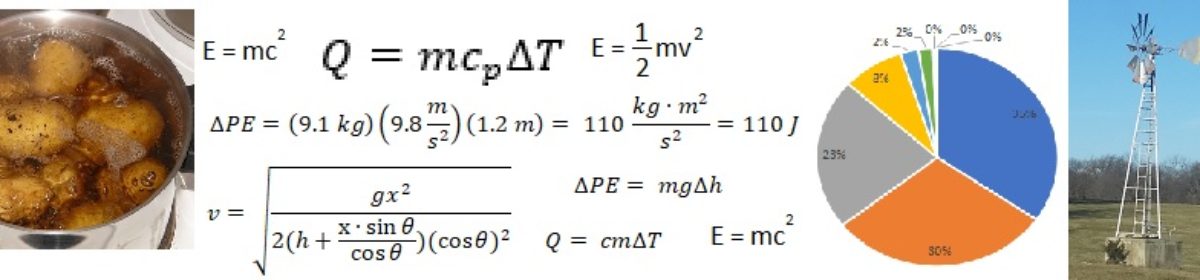Introduction
I enjoy backpacking in the great outdoors. As all good backpackers know, planning for access to water is a critical part of any backpacking trip. Backpacking is a lot of work and usually involves a lot of sweating, so keeping hydrated is necessary. However, maintaining a backpack as light as possible is also a key consideration, so one doesn’t want to carry a lot of water around. Thus, the usual strategy is to stay close to natural sources of water and bring a good water filter to allow for regular refills of water containers.
As a side note, how much work is a backpacking trip, you ask? Oh, at 100% efficiency, almost one million Joules worth of work would be required on a typical mountainous trail with about 1,000 meters of up and down (average 5% grade for a 20 kilometer, or about 12.5 mile, trip) during the full trip. (That calculation is simply the potential energy, which is the mass multiplied by acceleration due to gravity multiplied by the total elevation change). However, considering the body is probably only about 5% efficient in this case, the real energy use is approximately 20 million Joules, which is equivalent to a total of 5,000 food calories. That is just the extra calories of food energy “burned” during the trip in addition to the resting metabolic calorie burn of about 2,000 calories per day.
While on a backpacking trip some time ago, my brother and I took a day hike up a mountain and we knew we would not be near any sources of water for a good portion of the day. I had two water bottles with me on the backpacking trip, one un-insulated bottle and one insulated Thermos bottle. However, I didn’t have enough foresight to bring a smaller bag on the trip to carry around for the day hike, so all I had was a plastic grocery sack (I know, some Californians reading this post will cringe at those words), and thus I only took one of my water bottles. Unfortunately, it turned out that I selected the wrong water bottle to take with me.
You see, even though I tried to ration my water intake, I found I was out of water with several miles left to hike. Fortunately, we found some snow that I ate a good amount of, and also packed a good amount into my water bottle. I figured it could melt as we went along our merry way and I would soon have some nice cool water to drink. Well, that didn’t work out as well as I hoped because the snow was painfully slow to melt. Ironically, as we continued our hike, we ended up in a downpour that lasted a few hours. So I was soaked to the bone and dehydrated at the same time, and I still couldn’t get the darn snow to melt in my water bottle!
I had plenty of time during the hike to think about methods to melt the snow into water so I could drink it. Since it relates to energy, I thought I would share my findings here. The underlying assumption below is that conduction heat transfer through the insulated water bottle was negligibly low in the short term, which I think turned out to be a pretty good assumption. Also, a spreadsheet containing the calculations is available below.
Air Blowing Method
My first thought was to simply blow air into the water bottle’s straw and have it come out the little pressure release hole. It would work like any basic heat exchanger. I could transfer heat from the air I exhaled into the snow and cause it to melt. We should all be somewhat familiar with heat exchangers, as many of us have at least two in/near our dwelling places as part of an air conditioning system. One heat exchanger (located inside) allows the transfer of heat from the air inside the living space into a refrigerant, and the other heat exchanger (located outside) allows the transfer of heat from the refrigerant to the outside air.
We can use a simple equation to determine how much air I would need to blow through my water bottle straw to melt the snow. To formulate this equation, I simply use the fact that the amount of heat required to melt the snow must be the same as the amount of heat lost by the air as it travels through the snow in the bottle.

Where
- Q is the total amount of heat required to be transferred from the air to the snow, in Joules.
 is the total mass of air required, in kilograms.
is the total mass of air required, in kilograms. is the specific heat of air, which is 1.0 Joules per kilogram degrees Celsius.
is the specific heat of air, which is 1.0 Joules per kilogram degrees Celsius. is the change in temperature experienced by the air as it moves from my mouth to the exit hole, in degrees Celsius. I assume the air temperature goes from about 32 degrees Celsius when it exits my mouth to about 5 degrees Celsius when it exits the water bottle, for a change in temperature of 27 degrees C.
is the change in temperature experienced by the air as it moves from my mouth to the exit hole, in degrees Celsius. I assume the air temperature goes from about 32 degrees Celsius when it exits my mouth to about 5 degrees Celsius when it exits the water bottle, for a change in temperature of 27 degrees C. is the total mass of snow, in kilograms. My water bottle fits about 0.5 liters of water. I haven’t packed the snow in completely tight, so I have about 0.4 liters of snow at a density of about 0.6 kilograms per liter, for a total of 0.24 kilograms of snow.
is the total mass of snow, in kilograms. My water bottle fits about 0.5 liters of water. I haven’t packed the snow in completely tight, so I have about 0.4 liters of snow at a density of about 0.6 kilograms per liter, for a total of 0.24 kilograms of snow. is the latent heat of fusion ((Wikipedia link)) of water, which is 334,000 Joules per kilogram.
is the latent heat of fusion ((Wikipedia link)) of water, which is 334,000 Joules per kilogram.
Now all we need to do is solve for ![]() to obtain how much air it would take going through the “heat exchanger” to melt the snow. However, just knowing the total mass of air required is not very helpful. What I really want to know is how long it will take to get that mass of air through the water bottle. Thus, I need to know my respiration rate. I need to know how much air (in units of mass, or kg) I can blow through the bottle in a given amount of time. A typical adult respiration rate is about 6 liters of air per minute. However, when I am hiking with a backpack on I am exercising relatively vigorously, so I probably have a respiration rate around 50 liters per minute.
to obtain how much air it would take going through the “heat exchanger” to melt the snow. However, just knowing the total mass of air required is not very helpful. What I really want to know is how long it will take to get that mass of air through the water bottle. Thus, I need to know my respiration rate. I need to know how much air (in units of mass, or kg) I can blow through the bottle in a given amount of time. A typical adult respiration rate is about 6 liters of air per minute. However, when I am hiking with a backpack on I am exercising relatively vigorously, so I probably have a respiration rate around 50 liters per minute.
At an altitude of about 6,000 feet above sea level, the density of air is about 1.0 kilograms per liter. Using all of this information and solving for the time required to melt the snow (see the spreadsheet attached below if you want to follow all the math there), I obtain:

So it would take me about an hour to get enough air through the water bottle to melt the snow. However, this assumes that all of the air I exhale goes through the water bottle. I found that to be unsustainable in practice, as the water bottle restricted my exhalations enough that I would quickly run out of breath (remember, I am hiking on mountains wearing a 35-pound backpack). So I could really only get about one third of the air I exhaled through the water bottle. That results in about three hours to melt the snow. I am not that patient.
Potential Energy Input Method
My next thought was to use potential energy of the snow to melt it. The thought process is relatively simple. When I held the water bottle upright, there was a small pocket of air at the top. So if I turned the water bottle over, there would be stored potential energy in the lump of snow because the snow tended to stick to the bottom of the bottle.
As you will recall, potential energy is stored mechanical energy. In this case, it is the energy of the snow that can be realized as the snow falls the short distance from one end of the water bottle to the other. That potential energy must go somewhere as the snow falls. Initially, some energy becomes kinetic energy as the snow moves. When the snow stops against the bottom end of the bottle, about the only place for that energy to go is in the form of heat to the snow. Bingo! All I have to do is tip the water bottle upside-down enough times and I convert enough potential energy into heat energy to melt the snow!
So how many times do I need to invert the water bottle? Using the equation from the previous example, I already know it takes about 80,000 Joules of energy to melt the snow. So I just need to figure out the amount of potential energy for the snow in one time of inverting the water bottle.

It turns out the potential energy is about 0.1 Joules. So I only need to invert the water bottle 850,000 times. Assuming I can invert the water bottle once per second, this ends up being about 10 days. I am definitely not that patient.
Shaking Method
So what if I could speed up the potential energy process with some kinetic energy instead? Instead of inverting the bottle and waiting for the snow to fall in the water bottle, I can just orient the bottle horizontally and shake it back and forth instead and get a similar effect. I can increase the rate of impacts between the snow and the inside of the water bottle and maybe even increase the amount of heat added to the snow on each impact.
Consider the physics of what is going on inside the bottle as I shake it back and forth. As I shake the bottle in one direction, the bottle and snow move together. Then as I move the bottle in the other direction, the snow slams into one end of the bottle. This motion can be represented as a sinusoidal function. All I need to know are the amplitude of the motion and the period of the motion and I can fully describe the maximum velocity and acceleration of the motion. I assume the total range of motion is about 20 centimeters and I can move the bottle back and forth about three times per second. Thus, the motion of the bottle can be represented in this plot:

The position, velocity, and acceleration of the bottle can be represented by these equations (with just some calculus magic going on in the background):

In these equations, A is the amplitude of the motion, which is 10 centimeters, and T is the period of the motion, or the time it takes to complete a full cycle of motion, which is 0.333 seconds. The maximum velocity of the bottle (represented by the first portion of the velocity equation) is about 2 meters per second and the maximum acceleration (again, represented by the first portion of the acceleration equation) is about 35 meters per second squared, or about 3.5 times the acceleration of gravity. Considering a major league baseball pitcher can move a baseball from zero to 95 miles per hour in about 0.3 seconds (acceleration of about 15 times that of gravity), that seems like a reasonable value for acceleration.
It is the maximum velocity that I am interested in since that feeds into the calculation of kinetic energy for each cycle of bottle motion. It turns out that the kinetic energy in one impact of snow using the shaking method is about four and a half times the potential energy of the snow using the gravity method. In addition, the rate of impacts is about six times the rate for the gravity method. Thus, the shaking method is about 27 times as efficient as the gravity method. Still, it would take about nine hours to melt the snow using the shaking method, and that is assuming my arms don’t fall off before that point as a result of doing that much shaking. Nine hours is still too long.
Conclusions
In conclusion, none of the methods I tried turned out to be very effective for melting snow inside the insulated water bottle. The amount of time calculated for each method is shown in the table below. For comparison, I considered how long it would take the snow to melt if I had an uninsulated container sitting in room temperature (with heat transfer via convection of the surrounding air), using similar analysis to a previous post. It turned out that that method would actually result in a shorter time to melt the snow than any of the other methods.

So how did I end up melting the snow? The truth is, I never got the snow fully melted during the backpacking trip. When I found that the snow was not melting in spite of my best efforts, I decided to try another experiment. I decided to see how long it would take the snow to melt inside the bottle. Sure enough, when I arrived home approximately 24 hours after I put the snow in the bottle, there was still a small amount of snow remaining. So an insulated water bottle is pretty effective at keeping a drink cold. And if you are out backpacking and see some snow you want to use for drinking water, I recommend using a fire to melt it!
If you are interested in the calculations, see this spreadsheet: