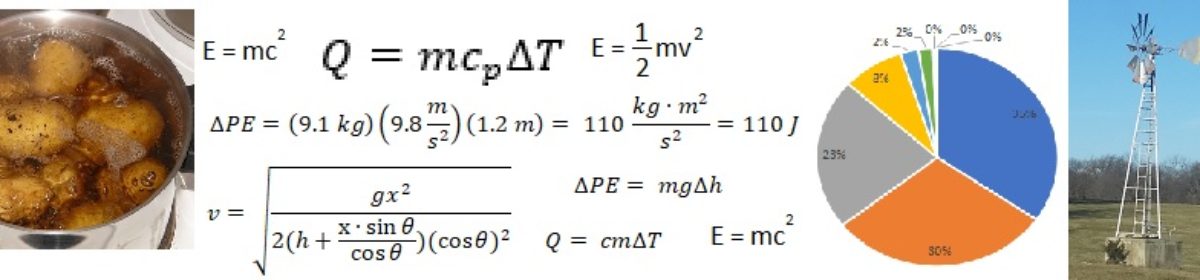How is energy measured? This is a great question, and deserves our attention in this post. As we consider how energy is measured, we will run into the most commonly used units of measurement for energy, joules (J), British thermal units (Btu), and watt-hours (Wh).
It turns out that measuring energy is difficult. Energy is not something that is readily accessible to our five senses. We can’t see, hear, touch, smell, or taste energy, at least not directly. Some properties of the world around us are readily available to our five senses, such as distance, mass and temperature. We can see physical dimensions that something has and can easily compare the size of one object to another. We can feel whether something is hot or cold.
Though we may not be able to see or touch something and immediately convert it to an exact dimension (as in, “I can see that this smartphone is exactly 15.3 centimeters long” or “I just walked outside and I can feel that the air temperature out here is exactly 10.7 degrees Celsius”), we can usually get pretty close (“this smartphone is between 10 and 20 centimeters long” or “the outside temperature is between 5 and 15 degrees Celsius”), and can almost always compare things like distance, mass, and temperature to know what things are bigger or hotter.
The same is not true of energy. We do not have an intuitive sense for energy like we do for other properties. Energy takes higher-level thinking. Consider these three examples:
- What takes more energy, lifting a 20-pound bag of potatoes from the floor to the kitchen counter or heating a small pot of water to boiling temperature?
- What takes more energy, driving your car to the grocery store or vacuuming the floors in your entire house?
- What releases more usable energy: one pellet of nuclear fuel (the size of an average marble) in a reactor in a nuclear power plant or 1,000 pounds of coal in a coal power plant?
Did you come up with the answers right away? How confident are you in your answers? Maybe you didn’t even hazard a guess because you just have no idea. I will share the answer to the first question later in this post (and the other two in a future post), but for now, let us consider why these questions may seem so difficult.
As mentioned, energy is not intuitively accessible to our immediate observations of the world around us. Thus, it wasn’t until the 1800s that the word “energy” was actually used in its modern sense of meaning, as a physical characteristic of an object or system that can actually be calculated and quantified. While it would be fascinating to dig into the history of the concept of energy, that is not my purpose here.
In order to understand how energy is measured, calculated, and quantified, it will be instructive to look at the meaning of some of the most widely used units of energy.
Units of Energy
The most widely-used unit of energy worldwide is the joule, named after James Prescott Joule, an English amateur scientist who lived from 1818 to 1889. A joule is equal to one kilogram meter squared per second squared, or one newton-meter. It is the amount of energy involved in moving with a force of one newton over a distance of one meter. The joule serves brilliantly within the overall International System of Units (also known as SI units) to measure various types of energy, including mechanical energy (kinetic or potential), chemical energy, and heat energy. The use of the joule is often the simplest method for completing energy conversions and calculations.
A watt-hour is a unit of energy that is often used to describe energy that is used over some period of time. A watt is a unit of power (amount of energy used over a given time period), defined as utilizing one joule of energy every second. A watt-hour is the amount of energy required to provide one watt of power over one hour of time. Since there are 3,600 seconds in an hour, one watt-hour equals 3,600 joules.
A British thermal unit (Btu or BTU) is the amount of energy that is needed (when added as heat) to raise the temperature of one pound of water by one degree Fahrenheit. The Btu is typically used (instead of the joule) in engineering calculations involving heating or cooling in the United States.
A separate unit in the English system of units is the foot-pound. It is defined in a similar manner as the joule, with one foot-pound being equal to the energy involved in moving with a force of one pound-force over a distance of one foot. However, the use of a pound-force can create confusion because the term “pound” is utilized to describe both mass and force in the English system of units. The foot-pound is used when mechanical energy (kinetic or potential energy) or work is involved.
Let us now use the above units to solve the first energy comparison question mentioned above: what takes more energy, lifting a 20-pound bag of potatoes from the floor to the kitchen counter or heating a small pot of water to boiling temperature?
Energy Comparison: Energy in the Kitchen
In the calculations of energy below, it is important to consider what quantity of energy is actually being calculated. In almost every process where there is a conversion of energy from one form to another, there is some inefficiency in the process. When lifting a bag of potatoes, some type of energy (if a person is doing the lifting, chemical energy in the form of glucose in the muscles or if a machine is doing the lifting, stored or produced electrical energy) is converted into potential energy as the bag is lifted up against the force of gravity.
The conversion of energy is never perfectly efficient. So when we talk about energy, we need to be careful and deliberate about what quantity we are actually measuring or calculating. Are we calculating the energy to perform a task in an ideal (imaginary) system or in the real world? The examples below will help to illustrate the difference.
So, what takes more energy, lifting a 20-pound bag of potatoes from the floor to the kitchen counter or heating a small pot of water to boiling temperature? First, we will determine the energy required to lift a 20-pound bag of potatoes to the kitchen counter.
As noted above, there is some inefficiency in the human body converting chemical energy stored in food to mechanical energy (work of physically lifting the bag of potatoes). In performing this energy calculation, we are ignoring that inefficiency and just considering the ideal (minimum) amount of energy to lift the bag of potatoes. That is, if the system for lifting the potatoes was perfectly efficient at converting stored energy into work to lift the bag of potatoes, how much energy would that system use?
Considered in this way, the energy required to lift the bag of potatoes is simply the difference in potential energy of the bag of potatoes between the countertop and the floor. The equation is: the difference in potential energy equals the mass multiplied by the acceleration of gravity multiplied by the change in height:

Let us first solve this equation using English units:

The result is 2,600 pounds-mass square feet per second squared. However, this unit is somewhat unwieldy. The more customary way to represent this amount of energy in the English system of units is using the unit of foot-pound. To understand how force and mass and mechanical energy are expressed in the English system of units, one would need to delve into the details of the units of the slug, pound-mass, and pound-force. Needless to say, it gets confusing. At any rate, expressed in foot-pounds, the energy would be 80 foot-pounds.
Now let us solve this equation using SI units:

A good approximation can be determined in this case without even using a calculator. Twenty pounds is approximately ten kilograms, and four feet is approximately a meter. The acceleration of gravity in SI units is about ten meters per second squared. So it is easy to calculate the energy in your head in this case and come up with about 100 joules, which would be pretty close to the right answer.
Now, we will determine the energy required to heat a small pot of water to boiling temperature. First, we need to make some assumptions. We will assume a “small pot” is about one liter (or 1.06 quarts) of water, and the temperature of the water is 50 degrees Fahrenheit (or 10 degrees Celsius).
Similarly in this calculation, there will be some loss of efficiency. If the heat source is a burner using a fuel (natural gas or propane), the gas will not burn perfectly, so there is still some chemical energy stored that is not converted to heat. With either a burner requiring fuel or an electric burner, not all of the heat will go into the water. Much of the heat is lost to the surrounding environment. Once again, for purposes of the calculation, we will assume we have a perfect system and no energy is lost in the conversion from chemical energy (fuel in a gas burner) or electrical energy (electric burner).
Thus, under the ideal system assumption, the energy required to heat the water is simply calculated as follows: heat energy equals specific heat capacity of the water multiplied by the mass of water multiplied by the change in temperature:

We will assume that the specific heat capacity of water is constant (even though in reality it varies with temperature), which is a pretty good assumption as it doesn’t change much in the temperature range we are looking at. First, using English units:

Since the definition of the British thermal unit was made for such problems, the calculation is fairly simple and the answer is 350 Btu’s.
Now, in SI units:

With the results from each calculation, we are ready to make some conclusions.
Conclusions
Hopefully, one of the first things you notice is that the energy involved in heating the pot of water (380,000 joules) is vastly greater than the energy involved in lifting up the bag of potatoes (110 joules). In fact, the potatoes would need to be lifted up to the countertop about 3,500 times to equal the amount of energy required to heat the pot of water. It sure is a good thing we have a burner to add that energy for us so we don’t have to add the energy mechanically. That would be a lot of lifting to get that water to boil!
Another item to notice is that we could immediately compare the values when we used SI units. The joule is an effective unit of energy when working with both mechanical energy and thermal energy. For the English units, however, we have two different units for the different kinds of energy. In order to compare the mechanical energy (80 foot-pounds) to the heat energy (350 Btu), you would need to know that there is a conversion factor of about 788 to convert foot-pounds to Btu’s. Thus, the 350 Btu’s is equivalent to 280,000 foot-pounds, which is a 3,500 times greater than 80 foot-pounds.
So, while using Btu’s or foot-pounds works just fine in some energy calculations, both of these units were made for specific kinds of energy and don’t easily relate to one another. I believe we are generally better off thinking about joules, an international standard that is easy to compare from one form of energy to another. The joule was designed to make sense as part of the overall SI system without getting messy and confusing like English units.
Another conclusion we can make is that while energy can be converted from one form to another, it is rarely very clean. There are always losses due to inefficiency. This is why experiments performed in the 1840’s to demonstrate the mechanical equivalent of heat (principally performed by James Prescott Joule) were so important and so revolutionary. The principle now known as conservation of energy (that energy is converted from one form to another such that energy is never lost in the process) was not obvious prior to those experiments. Energy is always “lost” in energy conversions, but it is never really lost in that it always goes somewhere.
Finally, as we already discovered, it takes a whole lot more energy to cook those potatoes than to get them up on the counter to prepare them. And not just by a little bit, but by a huge amount. This isn’t necessarily intuitive to us in daily life. But now you know the secret and are aware of the relatively enormous amount of energy required to change the temperature of water. So next time you are cooking on the stove, just think, that is a lot of energy going into that water!